Abstract
Background: BCL2, a key regulator of apoptosis, is aberrantly expressed in many hematologic malignancies, which can lead to pathologic cancer cell survival. BCL2 inhibitors have been shown to be safe and effective, resulting in their approval for the treatment of pts with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and acute myeloid leukemia. Treatment with the currently approved BCL2 inhibitor, venetoclax, can be limited by common gastrointestinal toxicities, neutropenia, and the emergence of specific BCL2 mutations around the BH3-binding groove causing resistance. BGB-11417 was developed as a potent and highly selective inhibitor of BCL2. It has shown antitumor activity superior to venetoclax in human acute lymphoblastic leukemia, mantle cell lymphoma (MCL), and diffuse large B-cell lymphoma (DLBCL) xenograft models (Hu, AACR 3077). BGB-11417 also has a favorable pharmacokinetic profile with excellent bioavailability and selectivity for BCL2 at concentration <1nM. Toxicology studies have shown a broad therapeutic index and tolerable safety profile.
The combination of a BCL2 inhibitor and a BTK inhibitor is tolerable with synergistic activity in CLL and MCL pts (Hillmen, J Clin Oncol 2019;37(30):2722-9; Jain, N Engl J Med 2019;30;380(22):2095-103; Siddiqi, ASH 2020 S158; Tam, N Engl J Med 2018; 378:1211-23). Zanubrutinib is a next-generation BTK inhibitor that has shown excellent activity and favorable toxicity in pts with CLL/SLL (Hillmen, EHA 2021 LB1900) and MCL (Tam, Blood Adv 2021;5(12):2577-85); with approval for treatment in MCL. Here we report preliminary results of the BGB-11417-101 trial (NCT04277637) in pts with non-Hodgkin lymphoma (NHL) or CLL/SLL treated with BGB-11417 monotherapy or in combination with zanubrutinib.
Methods: BGB-11417-101 is a phase 1, open label, multicenter, dose-escalation and expansion study. Pts with NHL or CLL/SLL are treated with BGB-11417 as monotherapy or in combination with zanubrutinib. For dose escalation, pts with R/R B-cell malignancies are enrolled in 1 of 5 potential dose levels of BGB-11417 (40, 80, 160, 320, or 640 mg once daily). All pts utilize a ramp-up to intended target dose that varies by disease type. Pts in the combination therapy arm receive zanubrutinib 320 mg daily beginning 8-12 weeks before BGB-11417 is introduced. Adverse events (AEs) are reported per Common Terminology Criteria for AEs v5.0. Dose-limiting toxicity (DLT; assessed from ramp-up through day 21 at intended daily dose), evaluated by Bayesian logistic regression model, will be used to determine the maximum tolerated dose (MTD).
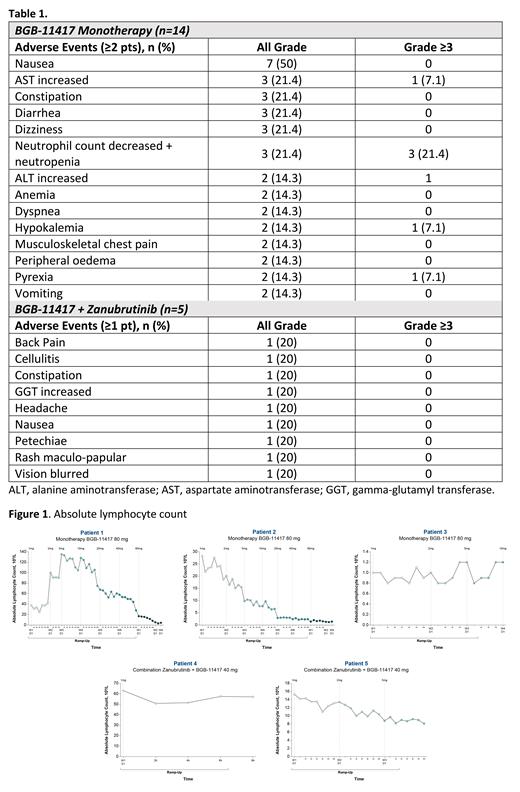
Results: As of 24 May 2021 (data cutoff) 19 pts had been treated; 14 pts with monotherapy (NHL: n=11; CLL/SLL: n=3) and 5 pts with combination (all CLL; 3 pts were still on zanubrutinib pretreatment; 2 had started combination treatment). Median age was 72 y (range, 50-86); median follow-up was 1.9 mo (range, 0.7-12.4); all pts were R/R with a median of 2 prior regimens (range, 1-4). No DLTs were observed in pts with NHL receiving BGB-11417 monotherapy (n=11) up to the 160 mg dose level. AEs across all dose levels occurring in ≥2 pts (monotherapy) or ≥1 pt (combination) are listed in Table 1. A total of 5 pts discontinued treatment (all NHL) due to disease progression (n=4; 2 at 40 mg, 2 at 80 mg) or lack of efficacy (n=1 at 40 mg). No pt discontinued due to AEs. Laboratory tumor lysis syndrome was observed in 1 pt with CLL and high tumor burden (resolved with no sequelae). Initial efficacy after 3-month restaging in pts with CLL/SLL demonstrated 1 partial response (monotherapy arm) at the first dose level tested. All pts with CLL/SLL who have completed ramp-up (n=2, both monotherapy) normalized absolute lymphocyte count (ALC). Marked decreases in ALC were observed in pts with CLL at doses as low as 1 mg (Figure 1).
Conclusion: Preliminary results suggest that BGB-11417 monotherapy is tolerable in pts with R/R NHL at the tested dose levels. Further assessment of safety and efficacy of BGB-11417 +/- zanubrutinib in CLL/SLL and NHL will be presented at the meeting, and evaluation in patients with treatment naïve CLL/SLL, R/R MCL, and R/R WM is planned.
Tam: AbbVie: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria; Loxo: Consultancy; Novartis: Honoraria; Pharmacyclics: Honoraria. Verner: Janssen-Cilag Pty Ltd: Research Funding. Lasica: Celgene: Other: Travel, Accommodations, Expenses; Janssen: Other: Education. Arbelaez: Amgen: Other: Travel, Accommodations, Expenses. Browett: AbbVie: Honoraria; Janssen: Membership on an entity's Board of Directors or advisory committees; MSD: Membership on an entity's Board of Directors or advisory committees. Soumerai: BeiGene: Consultancy, Research Funding; AstraZeneca: Consultancy; Adaptive Biotechnologies: Consultancy, Research Funding; AbbVie: Consultancy; GlaxoSmithKline: Research Funding; BostonGene: Research Funding; BMS: Consultancy; Seattle Genetics: Consultancy; TG Therapeutics: Consultancy, Research Funding. Hilger: BeiGene: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Fang: BeiGene (Shanghai) Co, Ltd.: Current Employment, Current equity holder in publicly-traded company. Huang: BeiGene: Current Employment, Current equity holder in publicly-traded company, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company, Divested equity in a private or publicly-traded company in the past 24 months, Other: Travel, Accommodations, Expenses; Protara Therapeutics: Current holder of individual stocks in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company). Simpson: Janssen: Research Funding; GSK: Research Funding; Pharmacyclics: Research Funding; Acerta: Research Funding; MSD: Research Funding; Roche: Research Funding; Celgene: Research Funding; Amgen: Research Funding; AbbVie: Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Research Funding; BeiGene: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Opat: Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GIlead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Mundipharma: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; CSL: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding; Monash Health: Current Employment; BeiGene: Research Funding; Sandoz: Research Funding. Cheah: Ascentage Pharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses, Research Funding; MSD: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Research Funding; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Lilly: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; TG therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Beigene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.
Zanubrutinib is an investigational agent and has not been approved for NHL or CLL/SLL in the US

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal